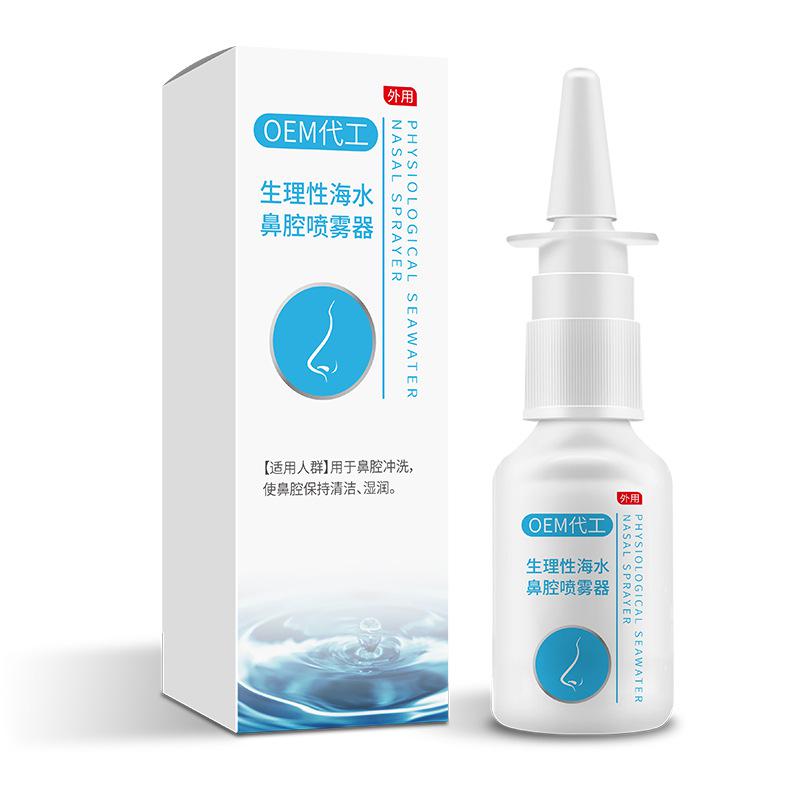
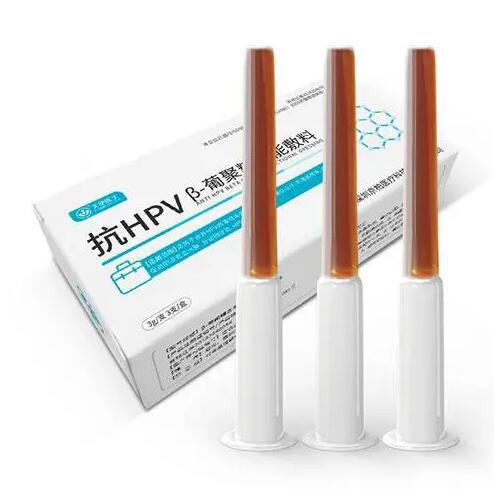
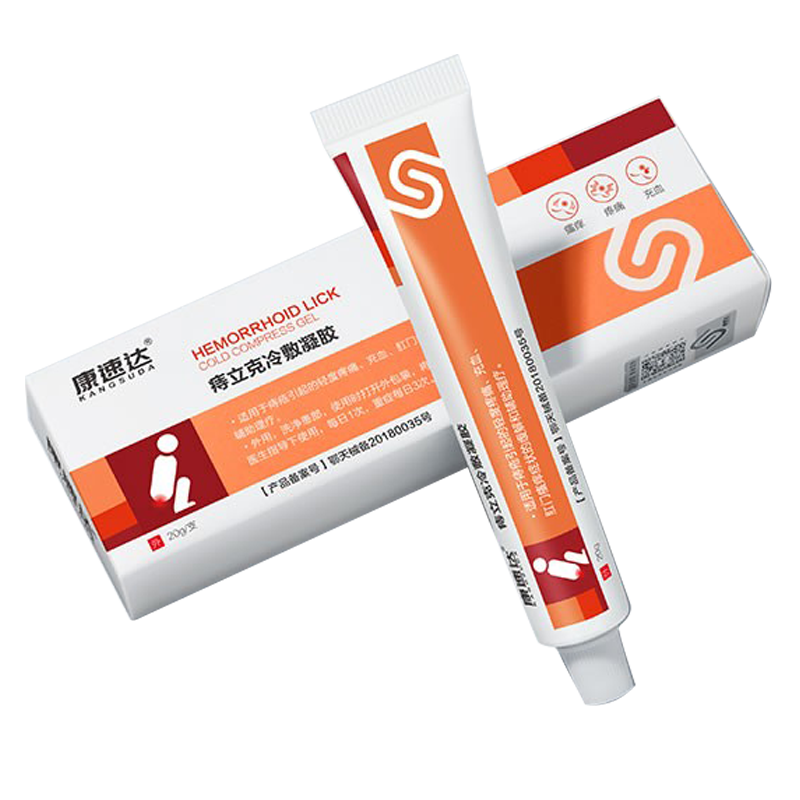
| 型号规格 | FD/PSG-W1812,FD/PSG-W1816,FD/PSG-W1818,FD/PSG-W1820,FD/PSG-W1823,FD/PSG-W2012,FD/PSG-W2016,FD/PSG-W2018,FD/PSG-W2020,FD/PSG-W2023,FD/PSG-W2412,FD/PSG-W2416,FD/PSG-W2418,FD/PSG-W2420,FD/PSG-W2423FD/PSG-L1812,FD/PSG-L1816,FD/PSG-L1818,FD/PSG-L1820,FD/PSG-L1823,FD/PSG-L2012,FD/PSG-L2016,FD/PSG-L2018,FD/PSG-L2020,FD/PSG-L2023,FD/PSG-L2412,FD/PSG-L2416,FD/PSG-L2418,FD/PSG-L2420,FD/PSG-L2423FD/PSG-Z1812-2104,FD/PSG-Z1812-2106,FD/PSG-Z1812-2304,FD/PSG-Z1812-2306,FD/PSG-Z1812-2504,FD/PSG-Z1812-2506,FD/PSG-Z1816-2104,FD/PSG-Z1816-2106,FD/PSG-Z1816-2304,FD/PSG-Z1816-2306,FD/PSG-Z1816-2504,FD/PSG-Z1816-2506,FD/PSG-Z1820-2104,FD/PSG-Z1820-2106,FD/PSG-Z1820-2304,FD/PSG-Z1820-2306,FD/PSG-Z1820-2504,FD/PSG-Z1820-2506,FD/PSG-Z1823-2104,FD/PSG-Z1823-2106,FD/PSG-Z1823-2304,FD/PSG-Z1823-2306,FD/PSG-Z1823-2504,FD/PSG-Z1823-2506FD/PSG-Z2416-2104,FD/PSG-Z2416-2106,FD/PSG-Z2416-2304,FD/PSG-Z2416-2306,FD/PSG-Z2416-2504,FD/PSG-Z2416-2506,FD/PSG-Z2418-2104,FD/PSG-Z2418-2106,FD/PSG-Z2418-2304,FD/PSG-Z2418-2306,FD/PSG-Z2418-2504,FD/PSG-Z2418-2506,FD/PSG-Z2420-2104,FD/PSG-Z2420-2106,FD/PSG-Z2420-2304,FD/PSG-Z2420-2306,FD/PSG-Z2420-2504,FD/PSG-Z2420-2506,FD/PSG-Z2423-2104,FD/PSG-Z2423-2106,FD/PSG-Z2423-2304,FD/PSG-Z2423-2306,FD/PSG-Z2423-2504,FD/PSG-Z2423-2506 |